


Determination of partial molar heat capacities and volumes of aqueous solutions at moderate temperatures
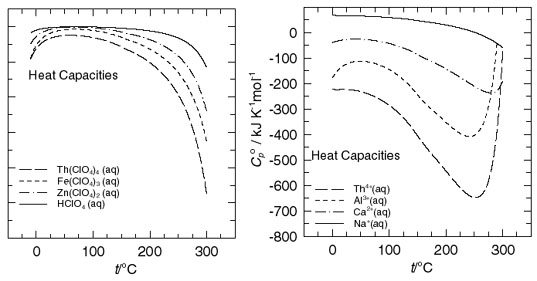
Using a modified Picker-type flow microcalorimeter connected along with a Picker-type vibrating tube densimeter, we are able to measure accurate volumetric heat capacities that in turn lead (along with measured partial molar volumes) lead to partial molar heat capacities of aqueous ions over a fairly moderate range of temperatures. This is an on-going research program for the investigation of the thermodynamic properties of aqueous species of importance to geochemical processes and specific environmental problems. To date this program has dealt with the determination of thermodynamic properties for various mineral solubility reactions as a function of temperature and pressure. Recent measurements have inolved actinide elements and complex ions, and work is currently underway on further analaysis of uranyl solutions, lanthanides, borates, and various more simple species such as sodium hydroxide, perchloric acid, and the iron(II)/iron(III) couple. These measurements have been applied extremely successfully for the prediction of high-temperature mineral solubilities using semi-theoretical models as guides. Future research is directed primarily toward the need for fundamental thermodynamic data on species important to model mineral dissolution and elemental transport, in addition to unique information under extremes of chemical variety, to aid in development of various models of ion-water interactions. Work is underway or planned involving the investigation of precious metals and their complexes, rare earth elements and complexes, selected actinide species (including uranium) and complexes, and transition metals and their hydroxides.
In collaboration with:
 |
Dr. Jamey K. Hovey Solution Calorimetry Lab. Leader. |  |
Prof. Terry Seward Geochemistry Group Leader. |

Currently in the calorimetry laboratory, we are developing improved methods for the precise measurement of thermal quantities under a variety of conditions. Work is currently underway for a moderately high-temperature specific heat calorimeter requiring an extremely small temperature step for measurements of super-cooled and very hot solutions using combined resistive, solid state, Peltier-effect, and other methods. Also we are currently developing an isothermal enthalpy calorimeter of mixing.
We have been fortunate to have inherited an LKB 8700 old-style enthalpy
calorimeter from Prof. Giorgio Anderegg, formerly of the ETH-Zurich. We have
upgraded this instrument with improved signal analysis and data collection,
although the basic workings remain the same. Early experiments currently
underway are examining the weak clusters formed between transition, lanthanide,
and actinide elements with chloride, sulphate, and nitrate ion near (0-50)
room temperature.
In collaboration with:
 |
Dr. Jamey K. Hovey Solution Calorimetry Lab. Leader. |  |
Prof. Terry Seward Geochemistry Group Leader. |
 |
Dr. Oleg Suleimenov Postdoctoral Fellow |
This page last updated on