


Models for the behaviour of electrolytic solutions are becoming more and more
based on the molecular nature of the solutions. To date, however, fundamental theoretical
models cannot predict the thermodynamic or thermaochemical properties of aqueous
electrolytic solutions from supercooled to supercritical because of the complex nature
of such calculations although great strdes are being made. In addition, however,
great advances are being made in gas-phase chemistry such as
fundamental cluster chemistry at Berkeley (especially for
moderately sized water clusters).
Our experimental program into the electrosprayed creation of ions is aimed at
helping to bridge the gap between our HPMS thermochemical
and spectroscopic measurements
on low-ligand number univalent ions to high-ligand number multivalent ions over wide ranges
of temperature. This program was born in cooperation with
Professor Kebarle at
Alberta and relies heavily on advances made in his and other laboratories.
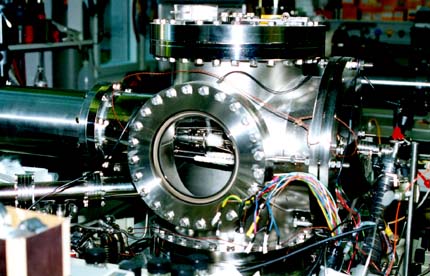
 We have modified a standard VG Trio-3 triple-quadrupole mass spectrometer by
replacing the source housing with our own very large high-pump capacity source
housing shown above. This is equipped with a 2300 L/s diffusion pump and a very
large working area. Electrospray experimental voltages come from our home-made electronics
distribution unit made by Sasha.
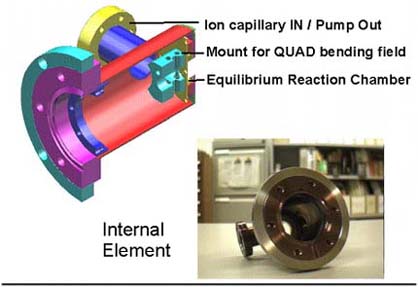
While we await for our Equilibrium Electrospray ion source being
machined since 1996, we are investigating the electrolytics
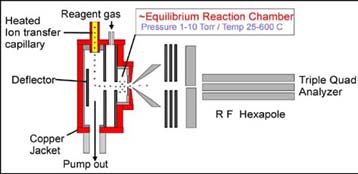
solutions using the simplified ion source shown schematically on the left.
Here instead of a "clean" manner in which to deliver the ions, we have adopted
the early Kebarle design of a simple deflector plate, focussing, and reaction
chamber. Early experiments are promising and underway. Inititial non-equilibrium
measurements carried out on an earlier version of the
ion source led us to design a siginificantly improved experiment with required
high ion sensitivity using a home made hexapole filter.
Our main experiment will employ 2 such units to bring ions gently into the
first mass filter and subsequent CID chamber. Watch here for results of our
new experiments currently in progress.
We have modified a standard VG Trio-3 triple-quadrupole mass spectrometer by
replacing the source housing with our own very large high-pump capacity source
housing shown above. This is equipped with a 2300 L/s diffusion pump and a very
large working area. Electrospray experimental voltages come from our home-made electronics
distribution unit made by Sasha.
While we await for our Equilibrium Electrospray ion source being
machined since 1996, we are investigating the electrolytics
solutions using the simplified ion source shown schematically on the left.
Here instead of a "clean" manner in which to deliver the ions, we have adopted
the early Kebarle design of a simple deflector plate, focussing, and reaction
chamber. Early experiments are promising and underway. Inititial non-equilibrium
measurements carried out on an earlier version of the
ion source led us to design a siginificantly improved experiment with required
high ion sensitivity using a home made hexapole filter.
Our main experiment will employ 2 such units to bring ions gently into the
first mass filter and subsequent CID chamber. Watch here for results of our
new experiments currently in progress.
Long term uses in addition to the elucidation of fundamental reaction schemes
are involved with the development of new theoretical approaches for the prediction of high
temperature and pressure properties of various
important geological species in water.
A few pics from the ESI lab
 |
Dr. Jamey K. Hovey Ion-cluster Laboratory Leader. | ||
 |
Prof. Terry Seward Geochemistry Group Leader. |  |
Dr. Oleg Suleimenov Postdoctoral Fellow (plans to start working with us soon .... |
This page last updated on